X chromosome inactivation is a fascinating biological process that plays a critical role in gene regulation, particularly in females who possess two X chromosomes. This mechanism ensures that one X chromosome is silenced, preventing an overload of gene expression compared to males, who have only one X chromosome. Recent breakthroughs, particularly from Jeannie T. Lee’s research, have illuminated how this inactivation is achieved through a unique gel-like substance that encases chromosomes, acting as a barrier and modifier. Understanding X chromosome inactivation not only unravels the complexities of genetic disorders like Fragile X Syndrome and Rett Syndrome but also unveils potential therapeutic avenues for restoring normal gene function. As researchers explore the therapeutic potential of reactivating inactivated genes, the hope for effective treatments for these conditions grows ever closer.
The silencing of one X chromosome in females, known as X-inactivation, represents a significant area of study in genetics, influencing our understanding of related conditions. This genetic regulation process impacts numerous diseases, particularly those linked to mutations on the X chromosome, such as Fragile X and Rett syndromes. In this context, researchers like Jeannie T. Lee are pioneering promising approaches that may unlock therapies benefiting those affected by these disorders. By manipulating the associated chromosomal architecture and exploring the ramifications for gene activation, scientists are tapping into new avenues that could revolutionize treatment strategies. The intricate dance of genetic material and therapeutic potential exemplifies how fundamental research in chromosome biology can lead to significant advancements in medical science.
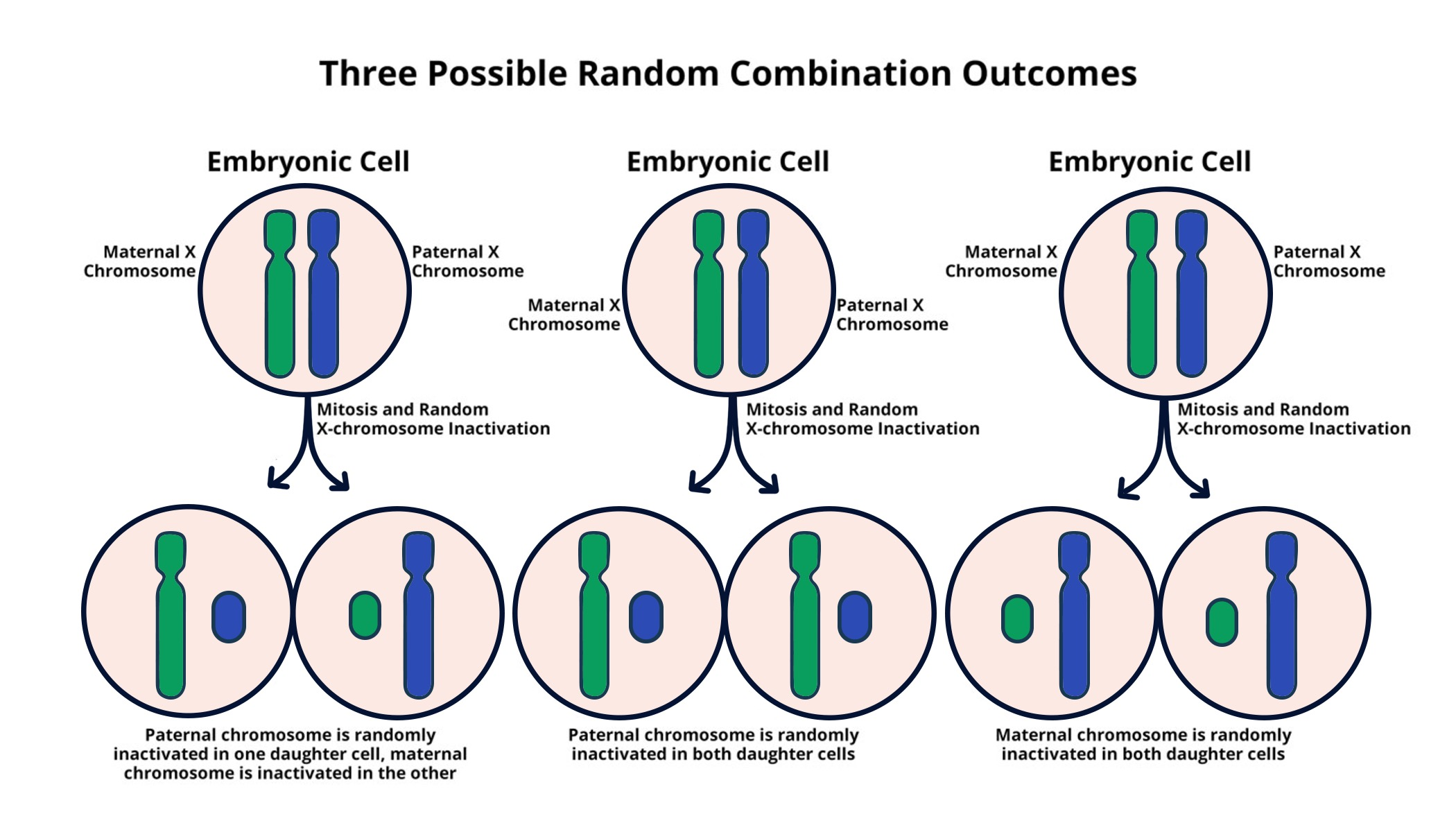
Understanding X Chromosome Inactivation
X chromosome inactivation (XCI) is a critical biological process that allows female mammals to silence one of their two X chromosomes, thereby ensuring dosage compensation between genders. This intricate mechanism allows females, with their pair of X chromosomes, to maintain a balance with males who possess only one X chromosome. The inactivation process is essential for normal development and function, and its understanding has profound implications for genetic disorders linked with the X chromosome. Jeannie T. Lee’s groundbreaking research has shed light on how this process operates at a cellular level, particularly the roles of specific molecules such as Xist that facilitate the inactivation.
In her studies, Lee and her lab members uncovered that the inactivation relies on a unique structure she describes as ‘chromosomal Jell-O’, a jelly-like substance that wraps around chromosomes. This substance plays a pivotal role in the spatial arrangement and accessibility of genes on the X chromosome. When Xist interacts with this chromatin structure, it not only engages in a molecular tug-of-war but ultimately leads to profound changes in the chromosomal architecture, rendering genes inactivated. The discoveries in this domain are not only fundamental but also hold potential therapeutic avenues for treating diseases such as Fragile X Syndrome and Rett Syndrome, where mutations on the X chromosome can lead to devastating outcomes.
The Therapeutic Potential of XCI Research
The intricate study of X chromosome inactivation (XCI) has opened doors to innovative therapies for genetically linked diseases. One such groundbreaking approach is the reactivation of inactivated X chromosomes to potentially utilize healthy gene copies that may be blocked by the inactivation process. Jeannie T. Lee’s lab is at the forefront of exploring this therapeutic avenue, having established experimental methods to release inactivated genes. This is significant for conditions like Fragile X Syndrome, where the healthy gene copy remains trapped within the silent X chromosome, preventing proper expression and leading to cognitive and developmental disabilities.
Lee’s research is not just focused on female cells; it has compelling implications for males affected by X-linked disorders as well. The findings suggest that despite not undergoing XCI, certain mechanisms can still silence genes on the X chromosome. Lee’s work has shown promise not only in the lab setting but also as a potential pathway for clinical translation. With ongoing optimization and safety studies, the hope is to move these compounds into clinical trials in the coming years, which could revolutionize treatment options for both Fragile X Syndrome and Rett Syndrome, elevating them from mere genetic curiosities to treatable medical conditions.
Genetic Disorders and the Impact of XCI
Genetic disorders, particularly those linked to the X chromosome, pose significant challenges in medical science. Conditions like Fragile X Syndrome and Rett Syndrome serve as poignant examples of how mutations on the X chromosome can lead to severe developmental issues. The role of X chromosome inactivation (XCI) in these disorders cannot be overstated; understanding the mechanisms behind XCI is crucial for developing effective interventions. As Jeannie T. Lee’s research indicates, the capability to selectively reactivate genes within an inactivated X chromosome could transform the treatment landscape for affected individuals, providing them with new hope.
Moreover, the insights gained through XCI studies highlight the delicate balance of gene expression in the human genome. The biological complexity of living organisms necessitates that researchers delve deeper into not only how XCI occurs but also its broader implications in genetic phenomena. With research continuously advancing, there lies an exciting frontier in genetics where disrupted pathways can potentially be reset, offering therapeutic avenues that were previously considered unattainable. The future of genetic disorder treatment hinges on such explorations, with Lee’s innovative work being a beacon of transformative potential.
Challenges in Reactivating Inactive X Chromosomes
Reactivating inactivated X chromosomes presents a unique set of challenges that require a sophisticated understanding of genetic mechanisms. The process is complicated by the cellular environment and the specific biophysical properties of chromatin, which Jeannie T. Lee’s research has illuminated. A key question remains: how can we effectively target inactivated X chromosomes without causing disruptions to the functional genes still present? While the prospect of restoring function to mutated genes is promising, ensuring the safety and efficacy of such treatments is paramount.
Lee’s lab has made remarkable strides in developing methods to navigate these challenges, with promising results that suggest it may be possible to harness the normal functioning of a gene while leaving unaffected genes intact. This careful approach minimizes side effects and enhances the therapeutic potential. Continued research into the mechanics of XCI not only seeks to address current obstacles but may also unveil novel strategies for treating a range of genetic disorders, making it an exhilarating time in the field of genetics.
Impact of Jeannie T. Lee’s Research on Future Therapies
The contributions of Jeannie T. Lee and her team are a testament to the impact that fundamental genetic research can have on future clinical applications. With decades spent deciphering the enigma of X chromosome inactivation (XCI), her work is now informing the development of targeted therapies for genetic disorders such as Fragile X Syndrome and Rett Syndrome. These conditions have previously suffered from a lack of effective treatment options. However, the emerging understanding of the roles played by factors like Xist and chromosomal structure opens up potential pathways for innovative therapeutic strategies that could significantly improve patient outcomes.
What stands out in Lee’s approach is her diligent focus on not just understanding XCI but translating that knowledge into practical applications. The laboratory’s efforts to develop compounds aimed at reactivating inactivated genes indicate a promising future where individuals affected by these genetic disorders may not only live better lives but potentially see profound improvements in their conditions. As research progresses toward clinical trials, the anticipation around Lee’s innovations heralds a new era in genetic therapy, where science moves from understanding to actionable treatment—a true revolution in the realm of genetics.
The Role of Xist in Gene Regulation
Xist (X-inactive specific transcript) plays a pivotal role in the regulation of X chromosome inactivation, making it a central focus of genetic research, particularly in understanding disorders associated with the X chromosome. This RNA molecule effectively triggers the silencing of one of the two X chromosomes in females, ensuring that gene dosage is correctly balanced between genders. Jeannie T. Lee’s work elucidates how Xist engages with the surrounding chromatin structure—referred to as ‘chromosomal Jell-O’—thereby facilitating a process that is not only critical for development but also sheds light on potential therapeutic interventions.
The investigation into Xist’s molecular interactions has revealed its capacity to modify the physical properties of chromatin, allowing for better access to genes that need to be silenced. This opens up intriguing avenues for research into whether manipulating Xist could help in reactivating silenced genes linked to various genetic disorders. By harnessing Xist’s functionality or employing it strategically, researchers could reprogram cells to bypass mutations that contribute to disorders such as Fragile X Syndrome and Rett Syndrome, thus creating a theoretical foundation for innovative treatments.
Future Directions in Genetics Research
The future of genetics research is being shaped by pioneering studies into X chromosome dynamics, particularly those led by researchers like Jeannie T. Lee. The exploration of X chromosome inactivation mechanisms not only promises insights into basic biological processes but also opens up routes for addressing complex genetic disorders. As the field progresses, the integration of advanced technologies such as CRISPR and gene editing tools may allow scientists to manipulate chromosomal behaviors, providing powerful means to treat diseases at their genetic roots.
Moreover, ongoing research into Xist and its modulatory effects on the chromatin environment presents exciting possibilities for developing novel therapeutic strategies. As Lee’s laboratory continues to push the boundaries of our understanding of X chromosome inactivation, the potential to translate these findings into clinical applications becomes more tangible. Future directions in genetics will likely encompass a more interdisciplinary approach, combining genetic insights with innovative therapy development to combat a diverse array of genetic disorders, ensuring a healthier future for those impacted by them.
Understanding Fragile X Syndrome and its Genetic Basis
Fragile X Syndrome (FXS) is a leading cause of inherited intellectual disability, primarily affecting males due to its linkage to the X chromosome. The disorder is caused by mutations in the FMR1 gene, leading to a lack of FMRP, a protein essential for neural development and cognitive function. Research into the genetic mechanisms behind FXS has intensified in recent years, particularly as scientists like Jeannie T. Lee aim to unravel the complexities of X chromosome inactivation and its implications for therapies. Understanding FXS at a molecular level could revolutionize how we approach interventions for affected individuals.
Recent advancements in the study of X chromosome behavior have provided insights into how the inactivation of the X chromosome can mask the presence of healthy genes. This raises the possibility that unlocking this process could allow for the expression of the FMR1 gene, restoring normal function and potentially alleviating symptoms associated with Fragile X Syndrome. By targeting the pathways involved in XCI, researchers hope to devise strategies that could reactivate silenced genes, providing a new direction for treating this challenging genetic disorder, thus inspiring renewed hope for those affected and their families.
The Significance of Rett Syndrome in X-Linked Genetic Disorders
Rett Syndrome is another profound example of an X-linked genetic disorder primarily affecting females, resulting from mutations in the MECP2 gene. Characterized by normal early development followed by a loss of motor and communication skills, Rett Syndrome presents a unique challenge for researchers focusing on both understanding its etiology and exploring potential therapies. Investigating the role of X chromosome inactivation (XCI) in the context of Rett allows scientists to contemplate novel approaches to gene therapy that could rescue functional gene expression.
Jeannie T. Lee’s work in understanding XCI suggests pathways that may be exploited to reactivate the silenced MECP2 gene in affected individuals. With genetic disorders like Rett Syndrome, where only one X chromosome is typically mutated, elucidating the dynamics of inactivation can lead to targeted therapies aimed not only at alleviating symptoms but potentially reversing the disorder. The intersection of X chromosome research and disease application is a rapidly expanding field, and the hope is that continued exploration will yield lasting benefits for patients grappling with Rett Syndrome in the near future.
Frequently Asked Questions
What is X chromosome inactivation and why is it important in genetic disorders?
X chromosome inactivation (XCI) is a biological process in which one of the two X chromosomes in females is silenced to ensure gene dosage balance between males and females. This mechanism is crucial for understanding various genetic disorders, such as Fragile X Syndrome and Rett Syndrome, where mutations on the X chromosome can lead to significant health challenges. Understanding XCI helps researchers develop potential therapies for these conditions.
How does Jeannie T. Lee’s research impact our understanding of X chromosome inactivation?
Jeannie T. Lee’s research has significantly advanced our understanding of X chromosome inactivation by elucidating the role of molecules like Xist and the surrounding chromatin, often referred to as ‘Jell-O’. Her findings indicate how these interactions can lead to effective therapeutic strategies for reactivating genes in disorders like Fragile X Syndrome and Rett Syndrome.
What therapeutic potential does X chromosome inactivation research hold for conditions like Fragile X Syndrome?
Research on X chromosome inactivation holds exciting therapeutic potential for conditions like Fragile X Syndrome by identifying ways to reactivate the healthy gene copy that is normally silenced. As researchers like Jeannie T. Lee explore the mechanisms of X-inactivation, they are paving the way for innovative treatments that could alleviate symptoms of genetic disorders linked to the X chromosome.
Can X chromosome inactivation findings help treat Rett Syndrome?
Yes, findings related to X chromosome inactivation are pivotal in developing treatments for Rett Syndrome. The mechanisms explored by researchers, particularly Jeannie T. Lee, suggest that therapies targeting the reactivation of silenced genes on the X chromosome could provide relief for affected individuals by restoring normal gene function.
What makes the mechanism of X chromosome inactivation complex?
The complexity of X chromosome inactivation arises from the fact that it does not merely involve the silencing of one X chromosome but also the intricate interactions with chromatin and RNA molecules like Xist. These processes involve a tug-of-war that affects gene accessibility and functionality, which are crucial for developing targeted therapies for genetic disorders.
How could restoring inactivated X chromosomes help in genetic therapies?
Restoring inactivated X chromosomes could help in genetic therapies by allowing the cell to utilize the healthy allele of a mutated gene. For diseases like Fragile X Syndrome and Rett Syndrome, where only one X contains the mutation, reactivation could potentially mitigate symptoms by leveraging the function of the healthy gene, leading to innovative treatment options.
What can we learn from the research on X chromosome inactivation by Jeannie T. Lee?
Jeannie T. Lee’s research on X chromosome inactivation teaches us about the fundamental biological processes that govern gene expression on the X chromosome. Her work reinforces the link between understanding genetic mechanisms and the development of therapeutic options, showing that insights gained from basic science can translate into treatments for serious genetic disorders.
| Key Points | Details |
|---|---|
| X Chromosome Inactivation | Females have two X chromosomes; one is inactivated to balance gene dosage with males. |
| Role of Xist | A gene on the X chromosome produces an RNA molecule called Xist, which modifies the surrounding chromosomal environment. |
| Mechanism of Inactivation | Xist interacts with a gelatinous substance around chromosomes, facilitating their silencing. |
| Potential Therapies | Research may lead to treatments for Fragile X Syndrome and Rett Syndrome by reactivating genes on the inactivated X chromosome. |
| Clinical Applications | Methods devised may enable the safe reactivation of certain genes while preserving healthy gene functions. |
| Future Directions | Further optimization and safety studies required before clinical trials can begin. |
Summary
X chromosome inactivation is a pivotal biological process that allows females to balance gene dosage between their two X chromosomes and the single X chromosome in males. This mechanism is critical for understanding genetic diseases linked to the X chromosome, as ongoing research uncovers potential therapies aimed at reactivating genes silenced during this process. Notably, advancements in the field led by researchers at Harvard Medical School offer promise in treating disorders such as Fragile X Syndrome and Rett Syndrome, which significantly affect many individuals. The journey to unlock the secrets of X chromosome inactivation has opened new therapeutic avenues, highlighting the profound implications of this genetic phenomenon.